- Asymmetric Carbon Atom Examples
- Asymmetrical Chemistry Definition
- Asymmetric Center Chemistry
- Asymmetric Carbon Atom In Glucose
A asymmetric carbon It is a carbon atom that is attached to four different chemical elements. In this structure, the carbon atom is in the center, linking the rest of the elements through it. The bromochloroodomethane molecule is a clear example of asymmetric carbon. The asymmetric carbon atom is a carbon atom in which all the valencies of the carbon atom is satisfied by the four different species. Asymmetric carbon atom: see isomer. Source for information on asymmetric carbon atom: The Columbia Encyclopedia, 6th ed. A chemical compound having the same number of each type of atom (same percentage composition and molecular weight) as another compound, but having different chemical or physical properties. Collins Dictionary of Medicine © Robert M. Youngson 2004, 2005.
Take a look at your hands. They are basically the same in style and function, but they differ completely in one respect--they cannot be exchanged for one another. If you put on your gloves, only your right hand fits into your right-hand glove. In this sense, your hands resemble 'right' and 'left' forms of molecules and your gloves resemble 'right' and 'left' forms of receptor molecules in a biological system.
The 'right' and 'left' properties of molecules derive from the fact that carbon atoms can form bonds with four other atoms in such a way that if the four atoms attached to the carbon atom are different, it is possible to draw two versions of the resulting three-dimensional molecule that are mirror images of each other. These are the right and left versions of a simple 'chiral' compound. They are exactly alike in every property except that they rotate plane-polarized light to the right or left, respectively.MIRROR IMAGE forms of lactic acid. |
The right and left properties of biological receptors derive from the chiral molecules from which they are assembled. One particularly relevant example of this type of receptor might be a protein whose subunits are amino acids. With the exception of glycine, the simplest amino acid, all the amino acid building blocks of living systems are chiral; furthermore, they are of the left-handed variety. A receptor protein is a large molecule formed of many of these left-handed subunits.
Receptors have a distinct three-dimensional structure whose surface consists of grooves and cavities. They can are capable of interacting only with three-dimensional molecules having a complementary structure (the 'hand in glove' analogy). But because of the complexity of these receptor molecules, these grooves and cavities sometimes may accept either right-, left- or 'no-handed' (achiral) molecules. Depending on which form of the molecule binds to the receptor, the biological results are often very different.
There are many examples of drugs in which the activities of the two forms, or isomers, are quite different. One example is the difference in pharmacological activity between the two isomers of the antidepressant sertraline (Zoloft). The right-handed version in this pair is a potent and selective inhibitor of the protein responsible for reuptake of the neurochemical messenger serotonin into cell membranes; the left-handed version has none of this activity.

By resolving this compound into its right/left components and using only the right-handed one as a drug, only half as much compound need be given, and potential side effects associated with the inactive enantiomer are avoided.
Asymmetric Carbon Atom Examples
James R. Paulson, a professor of chemistry at the University of Wisconsin at Oshkosh offers this perspective:
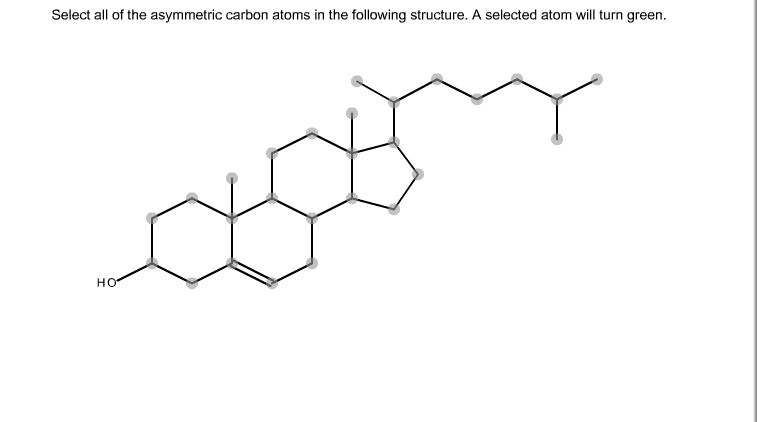
Sometimes two molecules look the same when you draw them on paper, but they are really different in three dimensions. This possibility arises whenever a carbon atom (which virtually always forms four bonds to other atoms) has four single bonds to four different groups. Because there are two ways that the four groups can be arranged around the carbon atom in three-dimensional space, and we call it an 'asymmetric carbon atom.'
Many of the molecules found in living things, including DNA and proteins, have asymmetric carbon atoms. For example, the simple sugar glyceraldehyde has an asymmetric carbon atom. So there are really two possible molecules, D-glyceraldehyde and L-glyceraldehyde. These two molecules are mirror images of one another, just as the left and right hands are mirror images of each other. Hence, we call them the 'right' and 'left' forms of the molecule.
Asymmetrical Chemistry Definition
It happens that for simple sugars only the D forms exist in nature. Yet it is of no significance that these are 'right-handed'--what is defined as left (L) and as right (D) is purely arbitrary--a human convention. The naturally occurring amino acids, from which proteins are built up, are in the L form.
Two mirror-image molecules interact identically with other molecules that are symmetric, but they interact differently with other asymmetric molecules such as proteins and DNA. This is because they have different shapes in three dimensions, and it causes them to have different biological activities.
Asymmetric Center Chemistry
This is perhaps best understood by means of an analogy with the right and left hands. Both hands interact identically with a symmetric object such as a pencil, a baseball bat or a tennis racket. But they interact differently with asymmetric objects such as a left-handed glove or a right-handed golf club.
Asymmetric Carbon Atom In Glucose
Asymmetry in molecules is very important in drug design, because drugs must interact with specific proteins or other molecules in the body. Drugs isolated from natural sources often consist of asymmetric molecules naturally. Synthesis of new asymmetric drugs in the laboratory is very difficult, however (see 'Chemical Asymmetric Synthesis,' by J. M. Brown and S. G. Davies in Nature, December 7, 1989). For example, the drug thalidomide exists in two forms, D and L--one is a powerful tranquilizer, whereas the other causes birth defects. In the 1950s and 1960s, when the drug caused a rash of horrible birth defects in England, it was not possible to separate the two. Researchers in pharmaceutical companies are now discovering ways to develop new drugs with specific handedness.
